The role of biomonitoring for exposure assessment in the REACH process
The Chemical Safety Assessment plays a leading role in the REACH procedure. In this respect, biomonitoring can make an important contribution.
The Regulation on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) has revolutionised the legislation on chemicals in Europe. Manufacturers, importers and downstream users are only permitted to manufacture, place on the market or use chemical substances which do not pose a risk to either human health or the environment.
The Chemical Safety Report plays a key role in the determination and description of possible risks. This report documents the characteristics of the chemical substance in question, its manufacturing and use as well as the associated risks which remain in the event of intended use, taking the defined risk mitigation measures into account.
The benchmark for risk assessment is the specific level of exposure below which the substance does not pose a risk to human health or the environment. The level of exposure can be determined using different methods: Firstly, by measuring the concentration of the substance in the air (external stress), and secondly, by determining the amount of the substance absorbed by the body or its metabolic products in bodily matter (internal stress). The latter usually occurs in the person's urine or the blood. It is therefore necessary to analyse the biological material of exposed individuals and to assess the health risk on the basis of the results.
In summary: Biomonitoring data provides valuable information for the exposure assessment in the scope of the REACH process.
The derived no effect level (DNEL) as an assessment criterion
This measurand describes the "derived level of exposure below which the substance does not pose any risk to human health".
The DNEL refers to the potential external impact of a hazardous substance, which can be absorbed by the body in different ways. It is also aligned to the relationship between the external dosage and a biological effect.
Types of external DNEL:
Differentiation between the duration and frequency of exposure:
- acute stress
- chronic stress
Taking the paths of exposure into account:
- inhalation uptake
- dermal uptake
- oral uptake
In particular need of protection:
- pregnant women
- sensitised persons
Instruments for the determination of exposure
Ambient monitoring:
Exposure to hazardous substances can be investigated by means of ambient monitoring. Research determines the dosage of a substance which is able to enter the body via a specific uptake pathway. This allows the direct comparison with the derived external DNEL while taking into consideration the uptake pathway.
Biomonitoring:
The concentration of the substance itself or those of an active metabolite at the target organ are responsible for the systematic effects of a hazardous substance. These can serve as biomarkers for the exposure and can also be measured in the appropriate bodily matter as part of the biomonitoring. As mentioned above, they are usually analysed in the blood or urine. The biomonitoring provides an assimilated measured value for the entire exposure which is independent of the uptake pathway and enables the assessment of the actual level of internal stress.
The derivation of a biomarker (DNELBiomarker)
It is not possible to directly derivate an external DNEL for assessing the results of the biomonitoring. A biomarker DNEL can, however, be derived on the basis of the toxicological contexts under consideration of the known biomonitoring limit values and/or risk assessments:
Case A
Clear dose-effect relationship between the biomarker concentration and the effect on a person or an animal
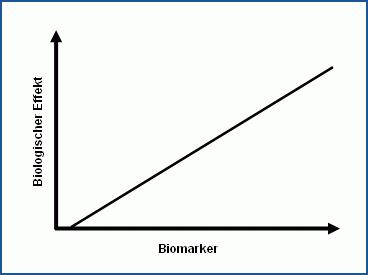
Person: possible application of a factor for the intra-species variation
Animal: application of factors for the inter- and intra-species variation
Case B
Clear relationship between the external exposure which correlates with the effects on the person or the animal and the biomarker concentration
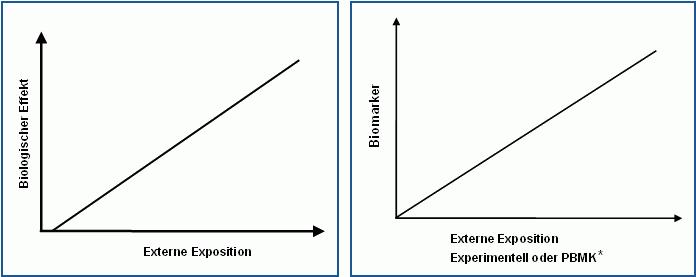
Person: possible application of a factor for the intra-species variation
Animal: application of factors for the inter- and intra-species variation
*pharmacologically based toxicokinetic model
Advantages of the biomonitoring
- Limitation of the number of safety factors
- Mostly a lower spread of biomonitoring data than with air measurement data.
- Validation of exposure estimates on the basis of external measurement data
- An overestimation of the exposure conditions is counteracted.
However:
- The biomonitoring data consists of individual values
- Drawing direct conclusions on the admission paths is impossible
- Environmental exposure which does not occur at the workplace is also measured
- It is necessary to take ethical and legal factors into account